LIF - Hologram Development Procedures
All pages in this lab
- Laser Induced Fluorescence and Raman Scattering
- Hologram Development Procedures
- Laser Startup Instructions
- Optics and the Spectrometer
- Troubleshooting
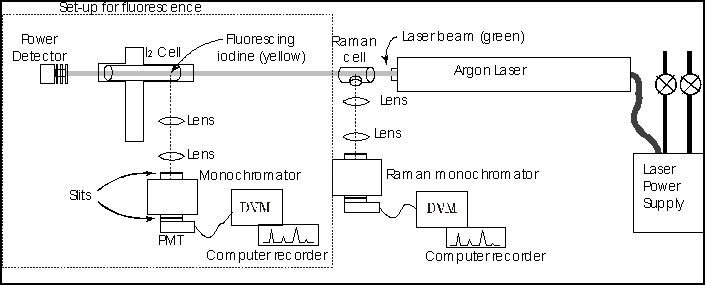
Right side of picture is Raman Setup
Equipment: Figure 5 is a block diagram of the apparatus. Identify each piece of equipment, and read the manuals on how to operate them.
- Position the iodine cell so that the laser beam passes through it end to end and the window faces the back spectrometer labeled Absorption/Fluorescence. Caution! This experiment uses a Class IV Laser, the highest classification of laser power. Even scattered radiation off of opaque surfaces can injure the eye. Be sure you know the safety issues before you start (watch the Laser Safety Video and discuss the apparatus with the staff). If there is any problem, immediately notify a staff member.
- Before you turn on the laser, check that there is nothing extraneous in the beam path and, most importantly, the beam is stopped by the beam stop at the end of the iodine cell track. Start up the laser according to the Laser Startup Instructions. Note: Make sure to wait a full 30 minutes for the laser to warm up and reach full power before adjusting the optics. If the power is still below 1.0 Watts after this time, ask for help in adjusting it. DO NOT ADJUST THE LASER MIRRORS YOURSELF.!!
- Position the spectrometer and the 15 cm lens in a straight line from the window in the iodine cell. Place the lens about 18.5 cm from the spectrometer slit (Use the two 50 micron slits for this part of the experiment.)
- By holding a white piece of paper in front of the spectrometer slit, you should be able to see the image of the laser line at the slit. You may have to shield out some of the background light with your hand. Also, there is a slit with white paper taped inside of it on which you can see the image of the line. Use one or both of these methods to see the image and focus it on the slit by adjusting the position of the lens. When you have focused the image, replace the 50 micron slit in the spectrometer.
- Turn on the photomultiplier tube and open the chart recorder program. Set the PMT to a Negative High Voltage around -1100 Volts.
- Turn the monochromator wavelength knob until you find the laser line. It will be near 514.5 nm.
- By moving the wavelength knob, you should be able to find smaller nearby peaks as well. With the wavelength set to one of these (fluorescence) peaks, adjust the position of the lens to maximize the signal. The higher your signal is, the more fluorescence peaks you will be able to observe. The more peaks you observe the better your analysis will be so take care with this.
- Set the PMT for a good signal-to-noise ratio. (The highest peak is the second one from the laser line.)
- Set the wavelength to 500 nm and begin scanning. You should see at least 15 lines without adjusting the settings. When it is necessary, adjust the photomultiplier tube to see as many lines as possible. You should find at least 24 lines. (The more lines you are able to identify, the less uncertainty you will have in your analysis so it is to your advantage to find as many as possible.)
Contents |
Raman Scattering
This section of the experiment requires by far the most finesse and patience. Expect some frustration and expect to spend some time finding a good signal.
You have seen how photons may interact with diatomic gas molecules to alter their energy states. In absorption and fluorescence, a molecule makes a transition from one stable state to another stable state by either absorbing or emitting photons matching the transition energy. But what if the energy of the incident light does not match any such transition? You will see that when you bombard diatomic gas molecules with monochromatic (i.e. laser, single-wavelength) light of an energy that does not match the energy of transition to a stable state, you may still observe light of frequencies other than the incident frequency emitted from the gas. This phenomenon is known as Raman scattering--when the wavelength of light is changed by scattering off of some substance.
The Heisenberg uncertainty principle tells us that $\triangle E\triangle t\ge\frac{\hbar}{2}$. So, if the uncertainty in the time is very small, the uncertainty in the energy may be large. This allows the energy of a molecular state to spread out for a very short time. Thus, a photon may combine with a molecule for a very short time in a "virtual state" of an unstable energy. The molecule must return to a stable state quickly but it does not necessarily have to return to the same state in which it began. When this happens, a photon is emitted with energy corresponding to the difference between the virtual or transition state and the final state. This energy may be the same as or different from the original photon energy. The energy of the emitted photon will be different from that of the original photon by an amount equal to the difference in energy between the original and final molecular states. In this experiment, we will observe rotational Raman scattering from diatomic oxygen and nitrogen. That is, we will observe Raman scattering in which diatomic oxygen and nitrogen molecules make transitions from one rotational state to another rotational state. The process involves two photons, each with one unit of angular momentum, so in order to conserve angular momentum, the selection rule for this process is $\triangle J=0 or \pm2$. (See Herzberg, pp. 91-93, for a description of Raman scattering; also, see Eisberg pp. 423-437)
The experimental arrangement is the same as that for iodine fluorescence, except that we are using a different cell built to withstand higher gas pressures and you will fill the cell with oxygen and nitrogen rather than iodine. The argon ion laser beam is focused in the center of the cell and light scattered at 90 degrees to the incoming beam is observed with a scanning spectrometer at wavelengths near that of the laser. See the apparatus diagram figure 5 above.
- Turn the laser on using the start-up procedure in the Laser Startup Instructions. Let the laser warm up while you continue with steps 2 through 7. The laser power is crucial in this part of the experiment. It will be extremely difficult to get satisfactory results without the laser powered to at least 1.0 Watt, so if it does not reach this power after 30 minutes of warming up, ask for help adjusting the mirrors.
- Fill the Raman cell with oxygen gas at a pressure of between 55 and 60 psi. (Ask for help the first time you need to fill the cell. The procedure for doing so is listed on a chart next to the gas canisters behind the lab bench for this experiment.)
- Use a ruler to measure the heights of all of the optical elements (lenses, spectrometer slit, laser beam--marked on outside of Raman cell to the right of the window). Adjust the lenses to within a millimeter or two of the height of the spectrometer slit and the laser beam. \*\* If the heights of the laser beam and the spectrometer slit differ by more than a couple of millimeters, ask for help. \*\*
- Place the two lenses as shown in the diagram. The first lens should be about 10 cm from the center of the Raman cell (line drawn on top of cell); the second lens should be about 10 cm from the spectrometer slit. Note: The flat side of the achromatic lenses should face the uncollimated light. That is, the flat side of the first lens should face the Raman cell and the flat side of the second lens should face the spectrometer for best results.
- Use a meter stick to check that all of the optical elements are centered on the optical axis from the Raman cell to the spectrometer slit. Note: It is important to make sure that the spectrometer slit is positioned directly in front of the Raman cell window. It should be about 9.5 cm from the edge of the optical bench. (Match up the line on the front of the spectrometer with the line on the optical bench.)
- Place a black board behind the lenses to shield out extraneous light. (See figure 9; wait to place the second black board in front of the lenses until you have them adjusted to maximize the signal.)
- Turn on the appropriate photomultiplier tube and make sure that it is connected to the HP 3478A DMM. Turn on the DMM and find the laser line.
- By adjusting the lenses carefully, you should be able to get the laser line up to at least 3 V on the DMM with the photomultiplier tube set around 1150 V. (This is just a rough guide; you do not need to use this setting.) When adjusting the lenses, remember that any adjustment that you make to one lens will affect the best position of the second lens. So move them together. For example, adjust the height of one lens to find a maximum and go a little beyond it until the signal decreases a again. Then, adjust the height of the other lens to raise it back up again. If the final signal (after both adjustments) is higher than the first maximum that you found, continue to move both lenses in the same direction. If the final signal is lower, then move both lenses in the opposite direction. Do this for all three directions of adjustment to find the maximum signal possible. Making small adjustments in the lens positions like this is known as "tweaking". The height adjustment is especially sensitive so try this one first. Unless you are very lucky, you will have to play with the optics for a while to find a good signal. Be patient. If you get frustrated, leave and come back and start over. NOTE: It is possible of course to find local maxima in the signal when the optics aren't really centered. DO NOT SUCCUMB TO THE TEMPTATION of moving the optics far from the center just because you are finding a signal there. The best signal and the only one that will really give you good results will be found very close to the ideal setup. Most importantly, you should be able to look down the optical axis and see the lenses in a straight line between the spectrometer and the Raman cell. See the troubleshooting guide in Appendix D if you are not getting anything at all or if you are having excessive difficulty finding a good signal. (Excessive difficulty in this case means at least an hour of effort.)
- When you have maximized the signal on the laser line, engage the motor on the spectrometer wavelength knob. Scan through a few nanometers. You should see peaks like the figures posted at the experiment.
- Set the wavelength to one of the peaks off of the laser line and adjust the lenses again to maximize the signal.
- When you are satisfied that you have maximized your signal, set the wavelength knob 4 or 5 nanometers below the laser line and take a spectrum. Again, the more lines you are able to find, the more effective your analysis will be. You should be able to find at least 10 lines on each side of the laser line. As usual, make sure to note carefully the wavelengths of the lines.
- When you have found a reasonably good spectrum with oxygen, fill the cell with nitrogen at 110 psi and take another spectrum. You should not have to adjust the lenses again. If the nitrogen spectrum does not come out well, it will be easier to refill the cell with oxygen and maximize the signal more effectively and then switch gases again than to maximize the signal with nitrogen in the cell because the nitrogen lines are harder to resolve and you may end up smearing them out if you are not exactly centered on a line when you are adjusting the lenses.
Please be careful! We want this experiment to be safe and fun. We are relying on you to follow procedures and use good judgment. For instance, before you start, make sure the beam path is free of extraneous objects. Never move objects in or out of the beam while the laser is on. And be mindful whenever you manipulate reflective materials around the laser beam: It only takes one unfortunate reflection to cause a lifetime of damage.
This experiment involves doing a bit of optics so you may need to brush up on a couple of ideas and learn a little about the spectrometers to get the best possible results. Watch the OPTICS video.
Lenses
A lens is just pieces of glass with curved surfaces such that the directions of incoming rays of light are changed according to Snell's law $n_1\sin{\theta_1}=n_2\sin{\theta2}$ at each surface. For any lens, there is a particular distance for which light from a point source at that distance from the lens becomes collimated (i.e. all rays parallel) upon passing through the lens. This distance is the focal length.
An object is in focus when all rays emerging from any point on the object converge together in a point on the other side of the lens. This occurs for a lens of focal length f when $\frac{1}{o}+\frac{1}{i}=\frac{1}{f}$, where o is the distance from the source (or, object) to the lens and i is the distance from the lens to the image. In other words, if the lens is a distance o from the source, the image will be focused a distance i from the other side of the lens.
The size of the image created is sometimes important. For example, in this experiment, we would like for the image to cover the spectrometer slit. Consider the three rays drawn below between object and image:
By noticing similar triangles, we see that the ratio of the height of the image to the height of the object (i.e. the magnification) is given by: $M\equiv\frac{i}{o}=\frac{h_i}{h_o}$. In a multiple lens system, the total magnification is simply the product of the magnifications for each individual lens involved: $M_{tot}=M_1\times M_2\times M_3\times...\times M_n$.
Spectrometer
In this experiment, you will use a spectrometer to measure the intensity of light as a function of wavelength. The spectrometer used in this experiment consists of multiple mirrors and a reflection grating as shown to the left. The mirrors collimate and refocus the light, and the grating spreads it out like a prism so that only light in a very small range of wavelengths will be focused on the exit slit at one time. When you turn the wavelength knob on top of the spectrometer, the grating turns and focuses a different range of wavelengths on the exit slit.
To get the best signal and the best resolution with the spectrometer, the most important factors are the following:
- The entrance and exit slits must be parallel--this affects mainly the resolution. It should not be a problem but if you are having excessive difficulty getting good results, you may want to check this.
- The full grating should be used. That is, it should be covered with the light. Since you can't see the grating itself, you have to make sure that it is covered by getting a large enough solid angle into the entrance slit. The spectrometer has an f number of 3.5 which means that the ratio of the distance from the final lens to the slit to the diameter of the lens must be less than or equal to 3.5 to make sure that the grating is filled. Basically, this means that your lens should be 15 cm or less from the spectrometer. We get around this in the absorption section of the experiment because the light is very intense.
To Turn on the Laser
- Ask a staff member for a pair of Laser Vision protection goggles. You must wear goggles while the laser beam is on.
- DO NOT TURN WATER ON FIRST !!! First PUSH and hold the water interlock box BUTTON while turning on the main water valve, located in the back left corner of the apparatus bench, turn main water valve CCW 90 degrees. The valve to the blue tank should be on with a Leave On sign labeled. Water should be flowing through the laser and out to the sink (located near the East wall in front of you as you face the laser). Make sure the water is flowing into the sink.
- Turn on switch
#28
in the circuit breaker panel. This panel is located on the wall between the Rooms 286A and 286B inside room 286.(to the right of the laser). Steps 4-11 refer to controls on the Spectra-Physics 265 Exciter Laser Power Supply located under the laser:
- Set the Meter Function switch to the 50 amp position.
- Set the Current Control to about mid-range (around 5).
- Set the Mode switch (to the right of the current control knob) to the left.
- Set the field control knob to 5 for, ten (10) minutes, then to its maximum value (full clockwise).
- Check that the "Water On" light and the three "Line" lights are on.
- Switch on the line switch.
- Wait 30 seconds for "Ready" light to be on.
- Press the "Start" button. There should be an output beam from the laser and the "Ready" light should be off.
- The output power of the laser can be tuned by adjusting the current knob on the power supply. You need at least 1 W of power to do the experiment (as measured on the front panel of the laser power supply meter). NOTE: the laser puts out ~ 1.4 watts of 514.5nm power. If you cannot attain that power level, see the staff.
- Run the Laser for stable output power for 30 minutes before starting to user the laser.
Note: During calibration or any time that the laser is not in use, turn it off by flipping the "line" switch down. Turn it back on using steps 4 through 12.
To turn off the laser when you are done for the day
- Turn off the 'line' switch on the laser power supply.
- Turn off breaker \#28 in the circuit breaker panel.
- Note You MUST turn off the main water IN-valve, CW 90 degrees, and then you can go home. The water will continue to run untill the Laser has cooled off. No overnight runs on this experiment. The water must be kept flowing until both inlet and outlet water pipes are the same temperature.
Gas Handling Procedures
Only adjust the gas if you have been trained to use these procedures by a staff member!
You will be releasing pure oxygen through the relief-valve, this is a volatile gas, please be sure that there are no open flames or smoldering embers nearby before you begin (Obviously this prohibits cigarette smoking).
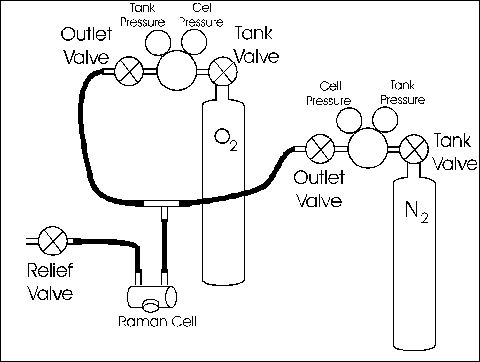
The procedures reference 'Tank-Valves' and 'Outlet-Valve' refer to the "Gas Flow Diagram" near the rear of this write-up.
- Close both tank-valves and outlet-valves.
- Open the cell relief-valve. The pressurized contents of the cell should begin venting through the relief valve with a gentle hiss about 5 seconds in duration. Leave the relief-valve open.
- On the tank of the gas with which you wish to fill the cell . . .
- Open the tank-valve and
- Open the outlet-valve
- Allow the system to flush for 5 to 10 seconds.
- Close the relief-valve
- The cell should pressurize to about . . .
- 55 ± 5 PSI for O2
- 110 ± 10 PSI for N2
- If it does not, do not attempt to adjust the pressure. Instead, ask for assistance.
- Close the tank-valve and the outlet-valve.